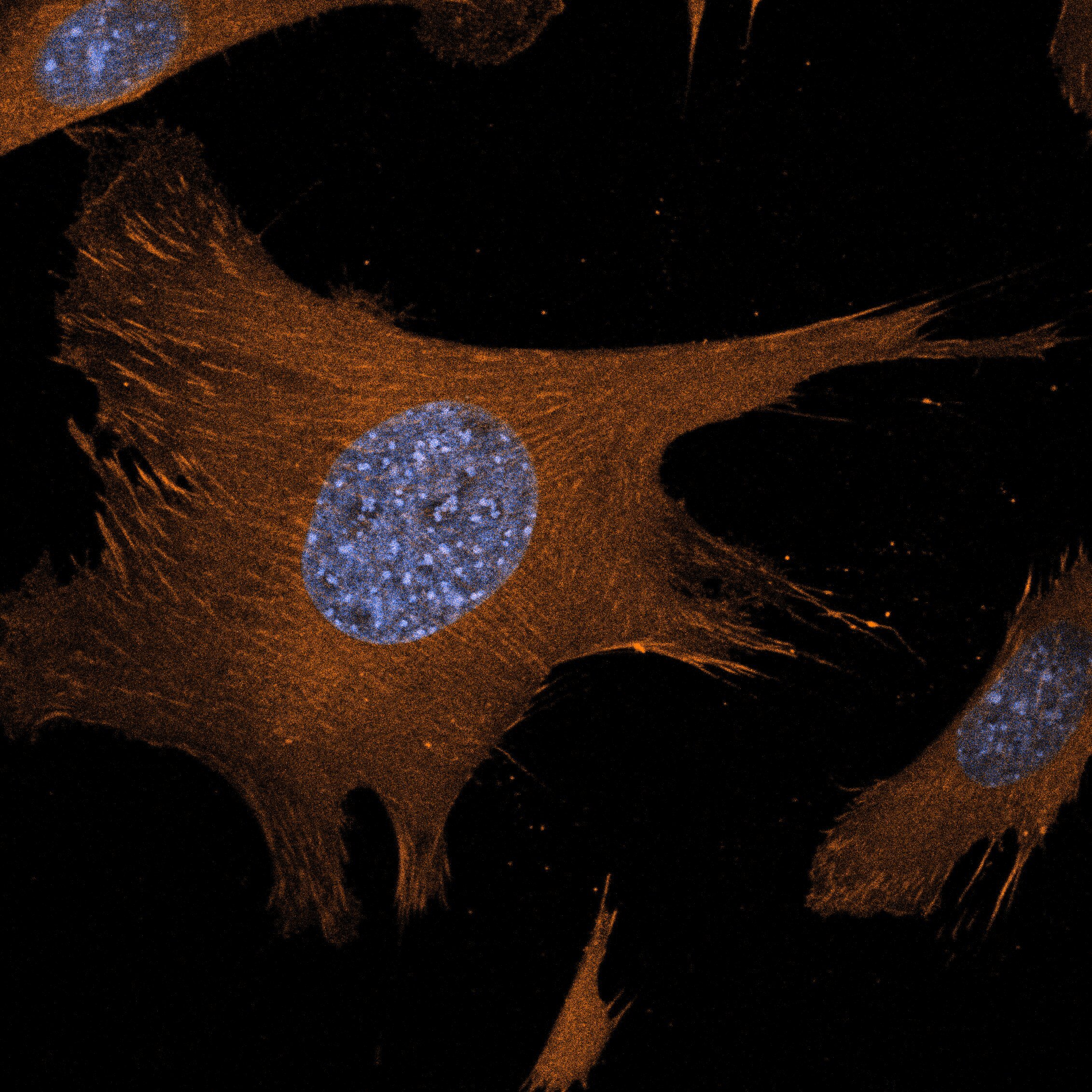
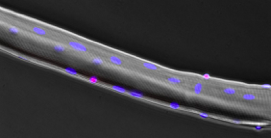
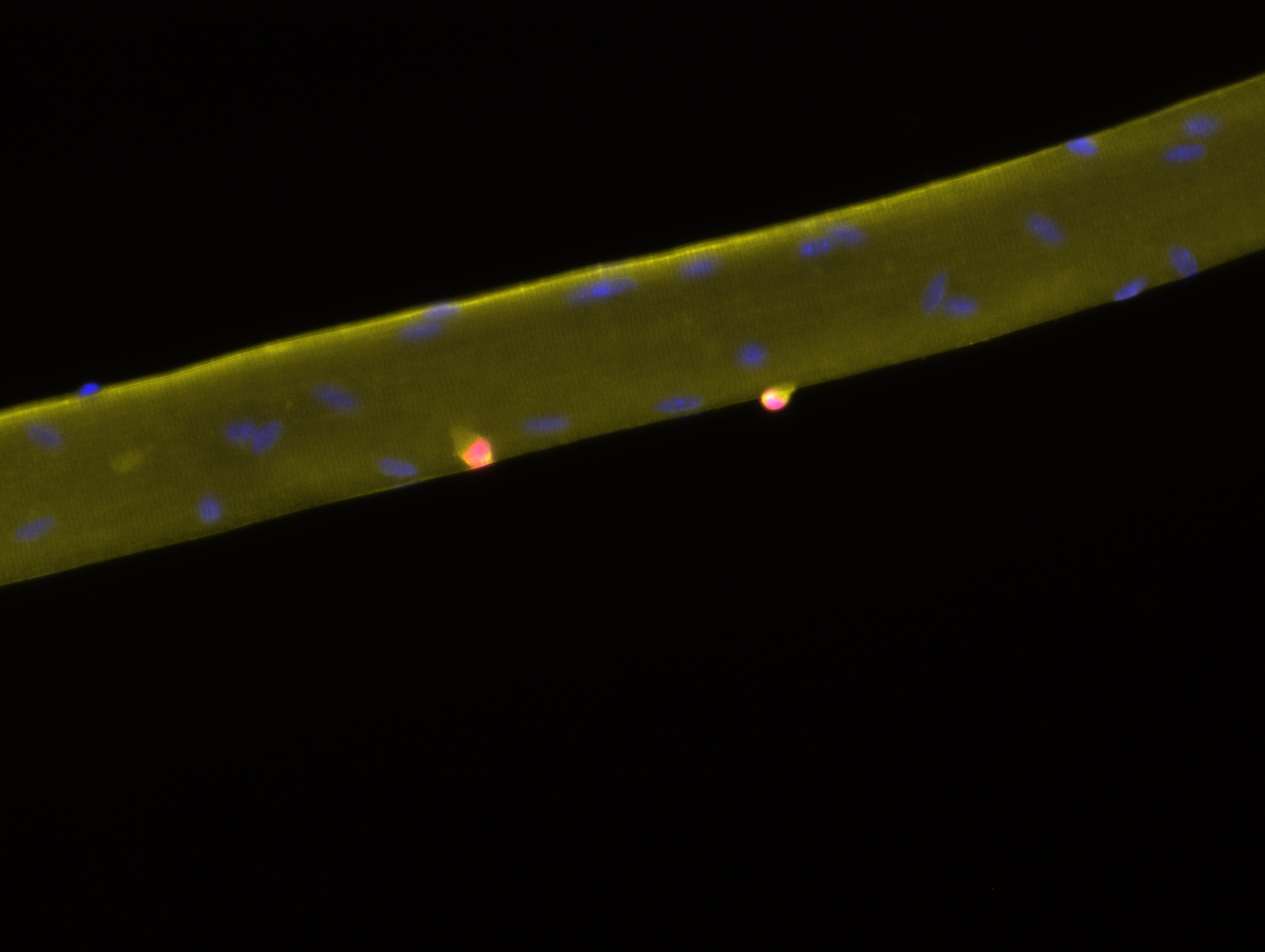
The native stem cell niche is a three-dimensional entity. While conceptually it is accepted that dimensionality is a critical feature of tissues that defines the location and timing of cellular events, understanding how dimensionality exerts such a powerful influence on stem cell biology is not well understood. Our team defines the interactions between muscle stem cells and their dynamic extracellular milieu that serve to orchestrate the elegant process by which a muscle stem cell switches between states of quiescence, activation, proliferation, differentiation, and self-renewal in health and in disease. Our specific emphasis is on evaluating how biomechanical stresses of the niche, like compressive forces, shear stress, or extracellular matrix stiffness, synergize with niche proteins to drive stem cell behavior. By quantifying in vivo biomechanical stresses presiding over the quiescent and regenerating adult skeletal muscle niche, and engineering three-dimensional culture models of skeletal muscle regeneration, we aim to uncover how the native three-dimensional tissue exerts spatiotemporal control over muscle stem cell fate.
Selected Publications
Moyle, MA, Cheng, RY, Liu, H, Davoudi, S, Ferreira, SA, Nissar, AA, Sun, Y, Gentleman, E, Simmons, CA, Gilbert, PM (2020). Three-dimensional niche stiffness synergizes with Wnt7a to modulate the extent of satellite cell symmetric self-renewal divisions. Molecular Biology of the Cell, 31(16): 1651-1821.
Li EW, McKee-Muir OC, Gilbert PM. (2018) Cellular biomechanics in skeletal muscle regeneration. Myogenesis in Development and Disease, Sassoon D (Ed.) Amsterdam, Netherlands: Elsevier.
Safaee H, Bakooshli MA, Davoudi S, Cheng RY, Martowirogo AJ, Li EW, Simmons CA, Gilbert PM (2017) Tethered Jagged-1 synergizes with culture substrate stiffness to modulate Notch-induced myogenic progenitor differentiation. Cellular and Molecular Bioengineering, 10(5): 501-513.
Davoudi S, Gilbert PM (2017) Optimization of satellite cell culture through biomaterials. Methods in Molecular Biology, E. Perdiguero and D. Cornelison (ed.), New York, NY: Springer, 1556: 329-341.
Gilbert PM*, Weaver VM*. (*Co-corresponding authors; 2016) Cellular adaptation to biomechanical stress across length scales in tissue homeostasis and disease. Seminars in Cell and Developmental Biology, 67: 141-152.
Morrissey-Scoot JD, Cheng R, Davoudi S, Gilbert PM. (2016) Biomechanical origins of muscle stem cell signal transduction. J. Molecular Biology, 428(7): 1441-54.
Cosgrove BD, Gilbert PM*, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SP, Blau HM*. (*Co-corresponding authors; 2014) Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nature Medicine, 20:255-64.
Mouw JK, Yui Y, Damiano L, O Bainer R, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, Hwang ES, Chen Y-Y, Weaver VM. (2014) Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nature Medicine, 20:360-367.
Gilbert PM, Blau HM. (2011) Engineering a stem cell house into a home. Stem cell research and therapy, 2(1): 1-9.
Gilbert PM*, Havenstrite K*, Magnusson KEG, Sacco A, Leonardi N, Nguyen N, Kraft P, Thrun S, Lutolf M, Blau HM. (*Equal Contribution; 2010) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science, 329(5995): 1078-81.
Lutolf M, Gilbert PM, Blau HM (2009) Designing biomaterials to direct stem cell fate. Nature, 462: 433-441.
Cosgrove BD, Sacco A, Gilbert PM, Blau HM (2009) A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation, 78(203): 185-94.